Explained: How Many Valence Electrons Does Calcium Have?

An atom has some electrons placed outside the energy level, and we call them valence electrons. Additionally, there are some interactions between atoms and these valence electrons. Moreover, they are quite far from the nucleus. So the other nucleus atom attracts them more compared to their nucleus. It is fascinating to know how many valence electrons does calcium have. Here, we explain the details of valence electrons and the valency of calcium:
Representation of Valence Electrons
Valence electrons are quite important. So we make simple diagrams to show the atoms with their valence electrons. Such simple diagrams are known as electron dot diagrams. Additionally, the dots in chemical symbols are the valence electrons. Atom can have only eight valence electrons up to the maximum level. Therefore, there cannot be more than eight dots of an atom.
Periodic Table
The periodic table shows the valence electrons of an atom along with its position. Also, the valence electrons increase from one element in groups 1 to 2 and 13 to 18. All the elements have similar valence electrons in the periodic table. Henceforth it represents that why all the elements have similar chemical characteristics in the same group.
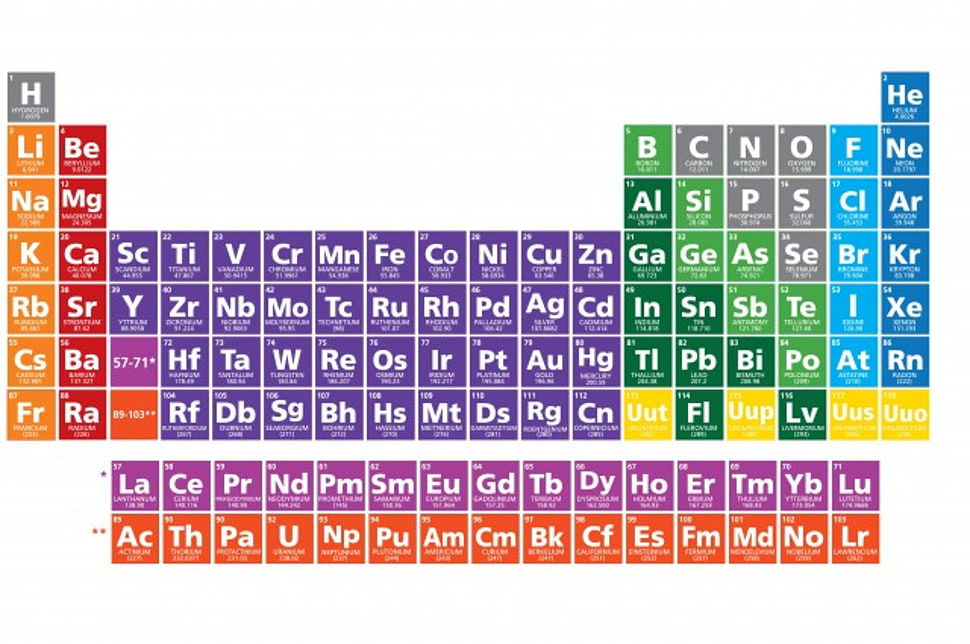
Periodic Table
Minimum and Maximum Valence Electrons in the Periodic Table
Hydrogen, Sodium, and Lithium have only one valence electron in the group. Likewise, Neon, Krypton, and Argon have eight valence electrons in group 8.
Electricity and Valence Electrons
The valence electrons determine the electricity of the atoms of an element. Moreover, the copper that is coated with plastic is a good electricity conductor. Therefore, the manufacturers use copper while making wires having electric current. Further, plastic having major carbon is used as insulation on wires because it cannot conduct electricity.
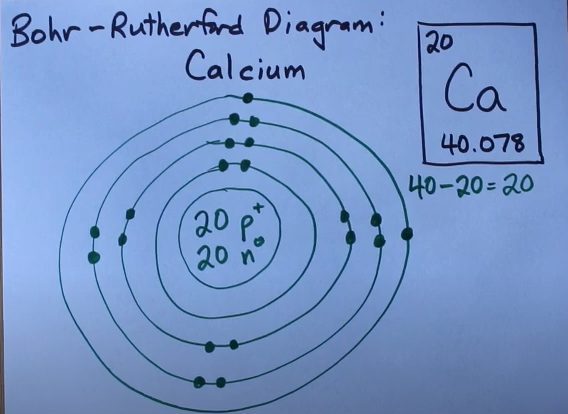
Valence electrons of calcium
Characteristics of Calcium
Calcium is a reactive element. It has a symbol of Ca. In addition, the atomic number of calcium is 20. It reacts with oxygen easily. The color of calcium is grey. You can easily find calcium in the maximum quantity in this world. It is amongst the top 3 metals that are easily available on the surface of the earth. Calcium is available in human beings as well. Bones have calcium. So it is an important particle of the human body. Some chemical elements occur in the body, and calcium is one of those chemical elements. Calcium carbonate is the significant compound of calcium that you can get from limestone. Moreover, it is helpful in digestion, muscles health, and circulation of the blood.
Calcium in the Periodic Table
Calcium is available in group 2 of the periodic table. It has 2 valence electrons. Having 2 valence electrons, calcium is choir reactive. It reacts with the element having 6 valence electrons that need to get two electrons. Oxygen in group 6 is that element.
Dot Diagram of Calcium Valence Electrons
Lewis dot diagram is the easy way to find out the valence electrons of calcium. The dot diagram helps in analysing the atoms and molecules’ structure for valence electrons.
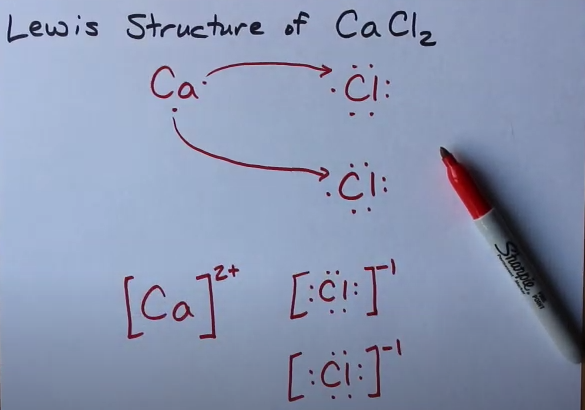
Dot diagram of calcium valence electrons
The Valence Electrons and Valency of Calcium
The valence electrons represent that how many electrons are available in the outer shell of the atom. Further, they are exact in number, and they cannot be more or less in number. Valency represents that how many electrons the atom can gain or lose in bond formatting to get stability. Valency changes and does not remain the same all the time. In addition, it varies because of variations in the reduction and oxidation conditions.
Calcium has 2+ valence electrons. It means that the valency of calcium is 2+. It has 2 electrons in the outer shell. The configuration is 2, 8, 8, 2. 2 valence electrons depict that calcium can get or lose 2 electrons. Moreover, it can be claimed that calcium gets stable by getting or losing 2 electrons. The feature of valency is one of the most significant features of the chemical element. This feature helps in gaining stability.
Rules to Determine the Valence Electrons of Calcium
There are two rules to find out the valence electrons of calcium such as the duplet rule and the octet rule. As per the octet rule, the atom can have a maximum of 8 electrons in the outer shell. As per the duplet rule, the atom can have a maximum of 2 electrons in the outer shell. Both of the rules are helpful in analyzing the valency of calcium. Furthermore, you can also use the periodic table to determine the valency of calcium.
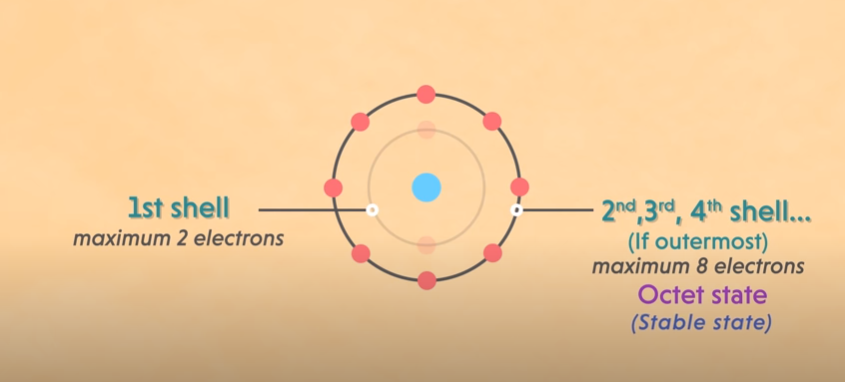
Rules to determine the valence electrons of calcium.
Steps to Determine the Valence Electrons of Calcium
You can find out the valence electrons of calcium by flowing these steps:
See the Periodic Table
Use the periodic table to get an idea about the calcium atomic number. When you see the periodic table, you will see that the atomic number of calcium is 20. Hence it means that calcium has 20 protons. Similarly, there is a feature of neutral calcium. Its number of protons is equal to the electrons.
See the Orbitals’ Arrangement
Now the next step is to analyze the orbitals’ arrangement of electrons. Its name is electron configuration. Further, it shows the details regarding the placement of electrons around the nucleus. So you will place the electrons in an arrangement showing the shape and energy of the orbital. To fill the s orbital, you require 2 electrons; to fill the d orbital, you require 10 electrons, to fill the f orbital, you require 14 electrons, and to fill the p orbital, you require 6 electrons. Furthermore, the placement of electrons around orbitals is made as per the Aufbau rule. According to this rule, first, you will fill the lowest energy orbitals. You can use the symbol ‘n’ for writing electron configuration.
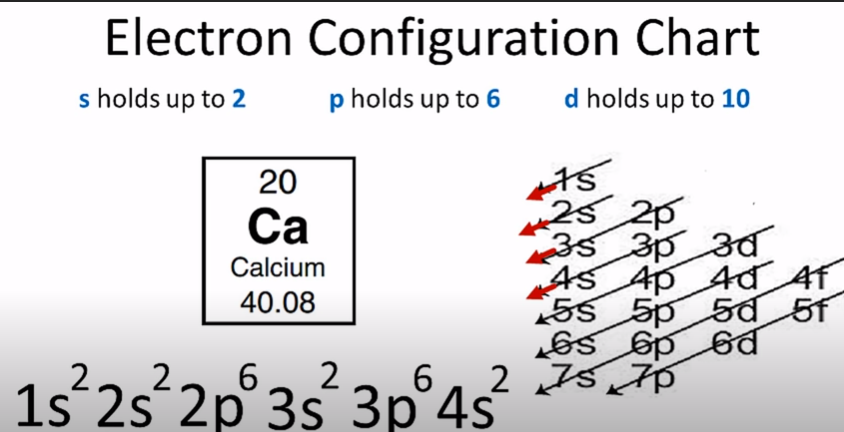
Electron configuration chart
Analyze the Quantum Numbers
Analyze the maximum number of principle quantum numbers to find out the valence shell.
See the Valence Electrons
As you know, the valence electrons exhibit how many electrons are present in the atom’s valence shell. Since calcium has 2 valence electrons in the valence shell, it is proved that calcium has 2 valence electrons.
How to Find Out the Valency of Calcium?
Valency represents how many electrons the atom can create a bond with the other electrons of atoms to be stable. In addition, the valency of calcium is related to the number of gains, losses, or sharing of electrons during bond formatting with the other atom. Calcium has 8 electrons in the outer shell; that’s why calcium is stable. The atoms having 4 electrons possess positive and negative valency. Moreover, the atoms having 8 electrons possess zero valency. Calcium loses 2 electrons then it goes towards stability. Henceforth, it is proved that the valency of calcium is 2.
Summary
How many valance electrons does calcium have? This question can be answered by finding out the number of valence electrons and valency of calcium. Furthermore, you can use the periodic table. Since calcium is present in group 2 of the periodic table, the elements in this group are known as alkaline earth metals having valency 2.
Similar reads: Does Crying Make Your Eyelashes Longer? Final Answer
-
Culture3 years ago
Viking Braids: Styles, Ideas and Method for Men and Women
-
Culture3 years ago
Cultural Integration: Definition, Examples, And Benefits
-
Culture3 years ago
Carmen Winstead: Full Story About the Terrorized 17 Year Old Girl
-
Fitness3 years ago
Best Royal Rumble Matches In WWE History
-
Culture3 years ago
Germany or Sweden: Which is Better For Lifestyle And Why
-
Culture3 years ago
German Mythological Creatures from German Folklore
-
Culture3 years ago
Health Triangle: Everything You Need To Know About it
-
Culture3 years ago
10 Best Examples Of Folk Culture